
#NAHCO3 MOLAR MASS HOW TO#

Legal How many cups of baking soda are in a lb? – Answers How many cups are in a 4 lb box of baking soda How many cups of baking soda equal 1 pound

See also Top 48 참나물 무침 황금 레시피 4520 People Liked This Answer How many cups are in a box of baking soda? –.Baking soda weight to volume conversion.1 Lb of Baking Powder to Cups Conversion.How many cups of baking powder in a pound?.How to Convert Cups to Pounds | Sciencing.Pounds to Cups Converter – (lb to c) – Inch Calculator.Pounds and Cups Calculator: Flour, Sugar, Butter and More.How many cups of baking soda are in a lb? – Answers.How many cups are in a 4lb bag of sugar?.How many cups are in a 5 pound bag of shredded cheese?.How many cups are in a pound of soda ash?.How much baking soda is in a standard box?.How many cups are in a 2lb bag of powdered sugar?.How many cups are in a 1lb box of powdered sugar?.How many cups are in a 5 lb bag of flour?.How many cups are in a 5 lb bag of sugar?.How much does a box of baking soda weigh?.Sana has been working at Whatsinsight since 2020 as a content writer. Related Links CO 2 Lewis Structure and Molecular Geometry HCN Lewis Structure| Step By Step Construction SO 2 (Sulfur Dioxide) Lewis structure N 2O Lewis Structure| Laughing Gas What is the Molar Mass of Nitrogen? Since CH 3COOH forms ice-like crystals below room temperature (16.6 ☌ or 61.9 ☏), it is also called “glacial acetic acid.” Important Links The name glacial acetic acid comes from the German name Eisessig (ice vinegar). It is used as a solvent for resins, paints, and lacquers.Īcetic acid is also called Glacial Acetic Acid in its anhydrous form.

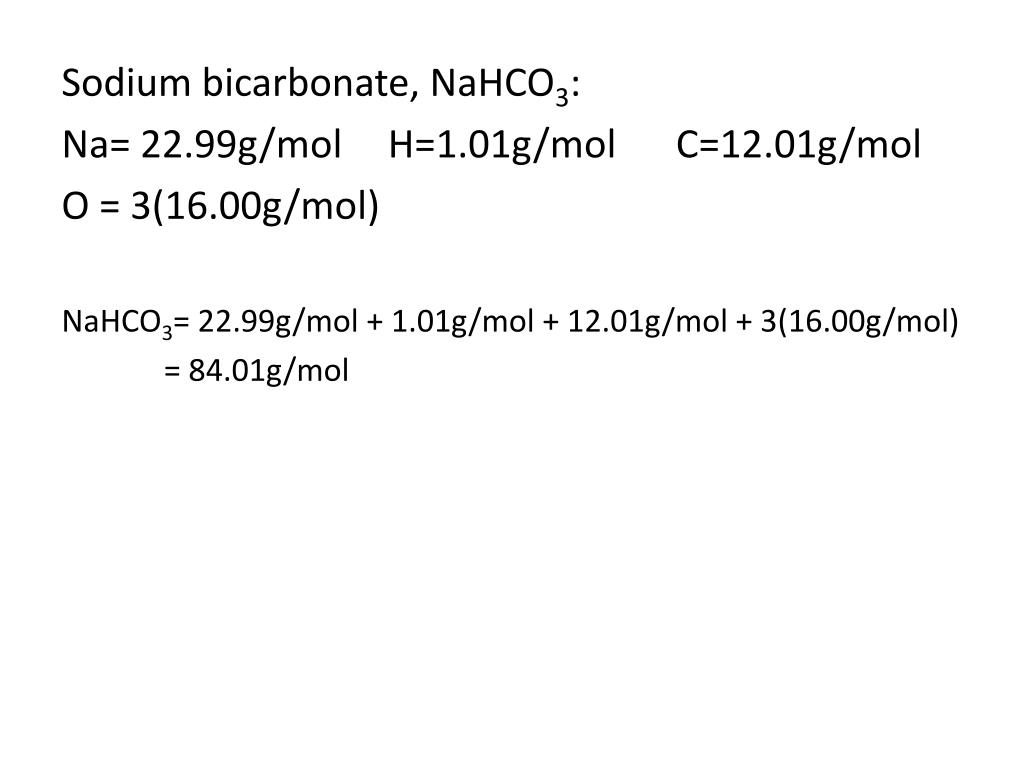
#NAHCO3 MOLAR MASS PLUS#
Two carbon atoms times 4 electrons, plus two oxygen atoms times 6 electrons, plus four hydrogen atoms times 1 valence electron equals 24 valence electrons. Step-2 (Determine the number of valence electrons in CH 3COOH ) Step-1 (Determine the number of valence electrons in individual atoms)Ĭarbon has 4, oxygen has 6, and hydrogen has 1 valence electron. In order to draw the lewis structure of acetic acid, we need to follow the following steps: Lewis Structure of CH 3COOH (Ethanoic Acid)įrom the structure, We can notice that CH 3COOH has 8 bonds (or 16 shared electrons) and 4 lone pairs (8 unshared electrons).


 0 kommentar(er)
0 kommentar(er)
